
Reduction of CD as a function of basic pH (Ca(OH)2 and NaOH) with a... | Download Scientific Diagram

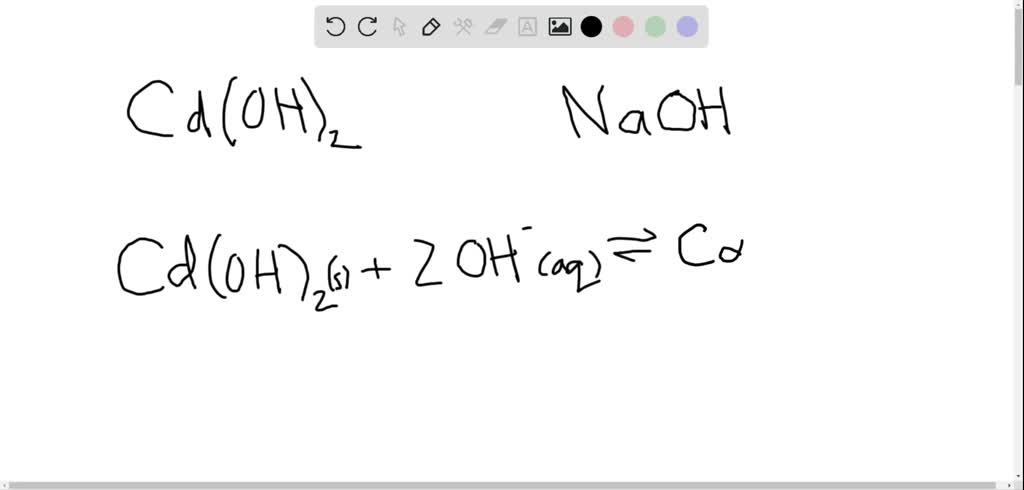
Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq, 0.01 M)|H2(g, 1 bar)|Pt(s) with Ecell = 0.0 V . If E^oCd^2 + |Cd = - 0.39 V , then Ksp of Cd(OH)2 is:
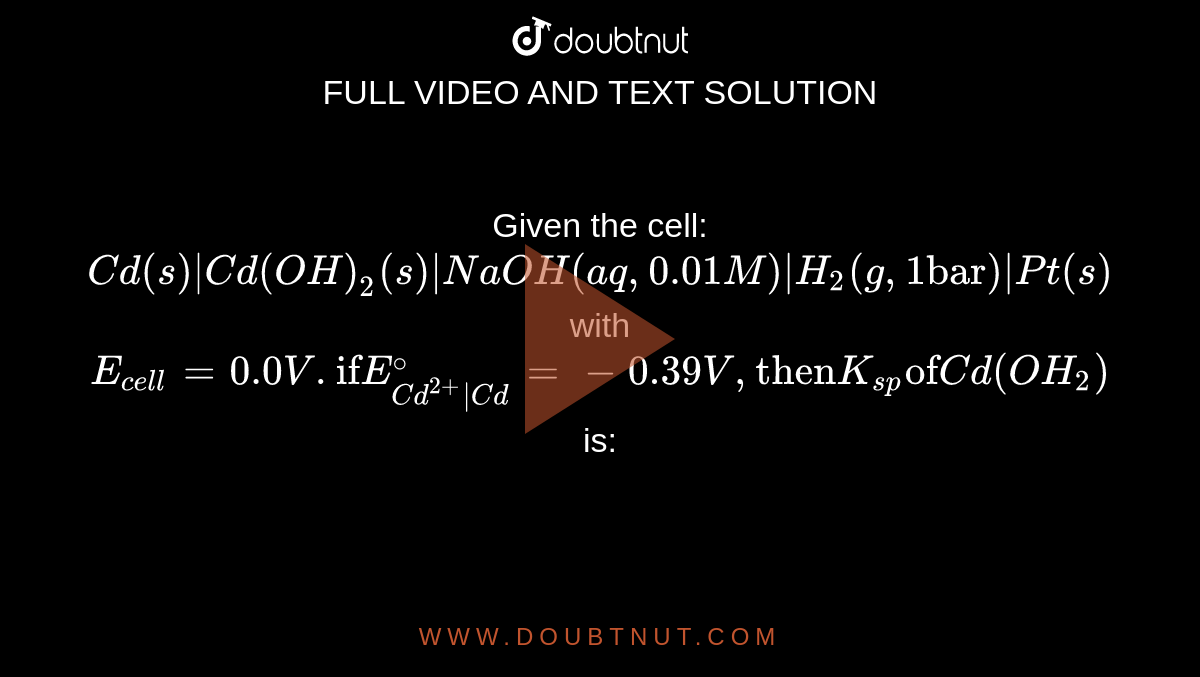
Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq,0.01M)|H2(g,1"bar")|Pt(s) with E(cell)=0.0V."if"E(Cd^(2+)|Cd)^(@)=-0.39V,"then"K(sp)"of"Cd(OH2)is:
Crystals | Free Full-Text | Effect of Different NaOH Solution Concentrations on Mechanical Properties and Microstructure of Alkali-Activated Blast Furnace Ferronickel Slag

Given the cell: `Cd(s)|Cd(OH)_2(s)|NaOH(aq,0.01M)|H_2(g,1bar)|Pt(s)` with `E_(cell)=0.0V.ifE_(Cd... - YouTube

Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq, 0.01 M)|H2(g, 1 bar)|Pt(s) with Ecell = 0.0 V . If E^oCd^2 + |Cd = - 0.39 V , then Ksp of Cd(OH)2 is:

OneClass: What mass of NaOH is needed to precipitate the Cd2+ ions from 35.0 mL of 0.460 M Cd(NO3)2 s...

Given the cell: Cd(s)|Cd(OH)_2(s)|NaOH(aq,0.01M)|H_2(g,1bar)|Pt(s) with E_(cell)=0.0V.ifE_(Cd^(2... - YouTube

Relationship between changes of extractability of Cd with NaOH and DTPA... | Download Scientific Diagram
The Equilibria of Cadmium Hydroxide in Acidic and Basic Media at 25° | The Journal of Physical Chemistry
No. 24 Studies on the Industrial Production of Monochlorotriazinyl-β-cyclodextrin and its Fixation to Textile Fibers | CycloChem Bio Co., Ltd.

a) CD spectra for ligand 4 in basic media (2 equiv. of NaOH). (b) CD... | Download Scientific Diagram

The principle on conductometric titration is based in the fact that during the titration, one of the ions is replaced by the other and invariably thses two ions differ in the ionic

Insights into the removal of Cd and Pb from aqueous solutions by NaOH–EtOH-modified biochar - ScienceDirect

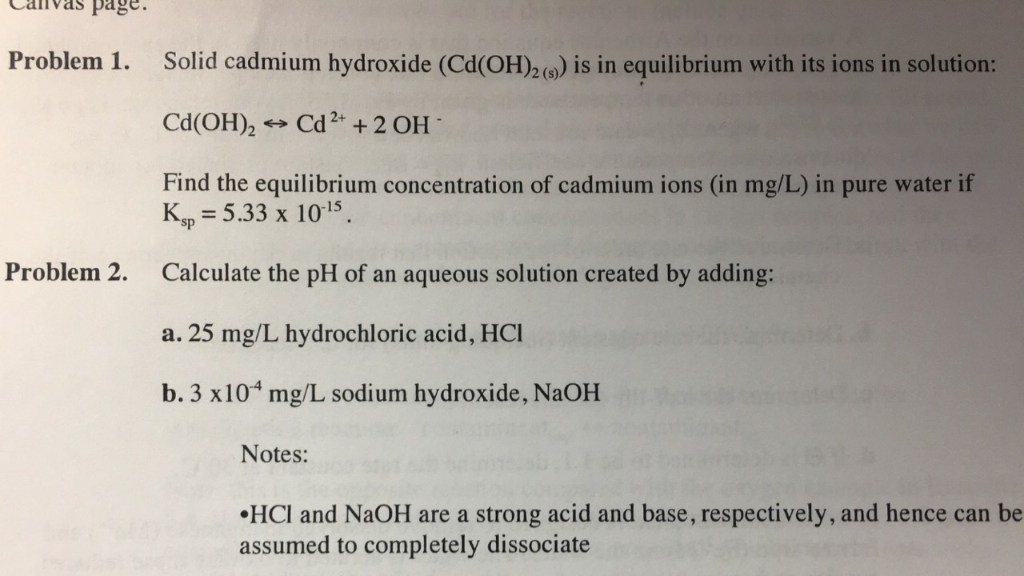
OneClass: 1. Let us assume that Cd(OH)2(s) is completely insoluble, which signifies that the precipit...


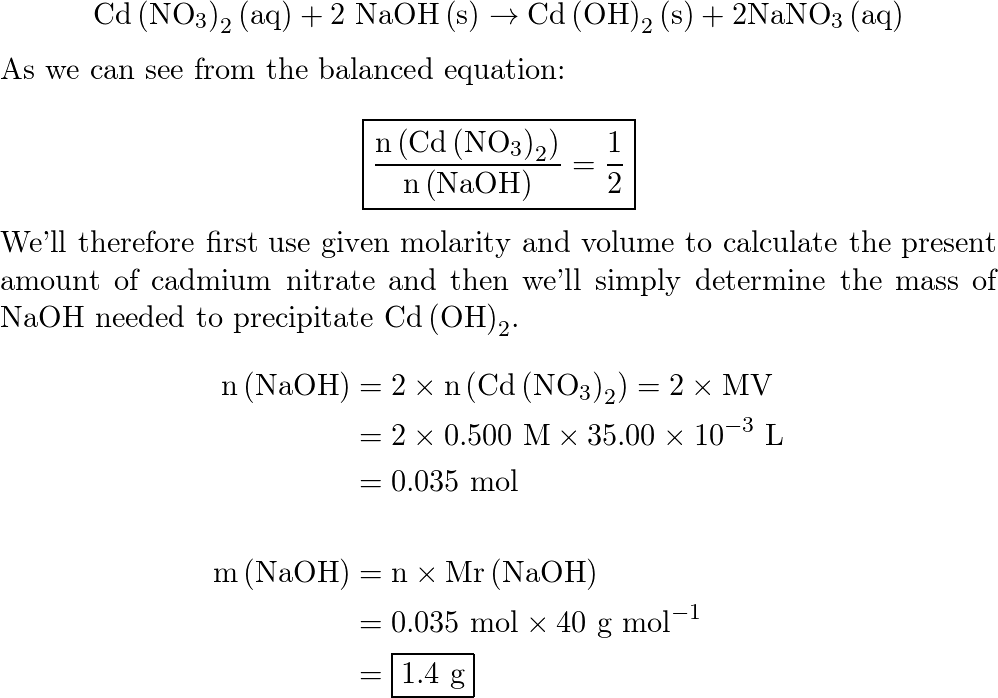

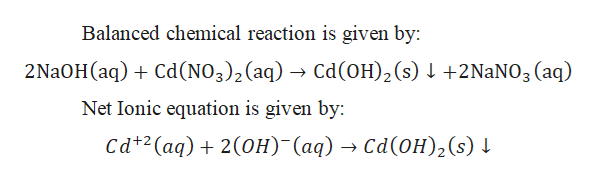
![ANSWERED] If 4 moles of NaOH react with 1 mole of C... - Physical Chemistry ANSWERED] If 4 moles of NaOH react with 1 mole of C... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/47412038-1658679986.4827924.jpeg)